| HOME | HELP | FEEDBACK | SUBSCRIPTIONS | ARCHIVE | SEARCH | TABLE OF CONTENTS |
|---|
|
|
||||||||
a Department of Medicine, University of Vermont, Burlington
b Division of Biometry and Risk Assessment, National Center for Toxicological Research, Jefferson, Arkansas
c Obesity and Diabetes Research Center, Department of Physiology, University of Maryland, Baltimore
d St. Luke's–Roosevelt Hospital and Columbia University College of Physicians and Surgeons, New York City
e Division of Geriatrics and Gerontology, Section of Applied Physiology, Washington University School of Medicine, St. Louis, Missouri
f Department of Physiology and Wisconsin Regional Primate Research Center, University of Wisconsin, Madison
Eric T. Poehlman, Given Building C-247, University of Vermont, Burlington, VT 05405 E-mail: epoehlma{at}zoo.uvm.edu.
| Abstract |
|---|
|
|
|---|
AN issue of major importance to gerontologists is whether changes in exercise behavior and/or body composition can result in life-span extension (i.e., either decrease mortality acceleration with age or change the intercepts of the survival curve). Panel 4 examined the role of changes in physical activity and body composition as possible modulators of altering life span. One broad question of interest generated by our panel is whether changes in exercise and body composition elicit changes similar to those found in dietary restriction (DR). A second question is whether changes in exercise and body composition can be used to either replace or enhance the beneficial effects of DR in humans. Given this broad area of research and the complexity of the interaction between exercise, body composition, and DR, it is not our goal to be exhaustive. We will, however, examine present evidence in experimental animals and humans regarding the specific contributions of each of these factors to altering life span and age-related pathologies. We will also discuss future areas of research to clarify these issues.
| Studies in Rodents |
|---|
|
|
|---|
In Goodrick's first study, in which wheel running began at age 1.5 mo, average longevity was 3–4 mo greater, and maximal longevity was  3 mo greater in the runners than in sedentary controls (5). In this study the sedentary controls were also short lived, with an average age at death of 631 days for the sedentary male rats. Average longevity for healthy, pathogen-free rats is in the 750- to 950-day range, depending on the strain. In another study, in which rats were placed in voluntary running wheels at 10.5 mo or 18 mo of age, Goodrick and coworkers found no increase in longevity of the animals given access to running wheels (6). Four subsequent studies on specific-pathogen-free Long Evans rats given free access to voluntary running wheels have confirmed that wheel running increases average survival by
3 mo greater in the runners than in sedentary controls (5). In this study the sedentary controls were also short lived, with an average age at death of 631 days for the sedentary male rats. Average longevity for healthy, pathogen-free rats is in the 750- to 950-day range, depending on the strain. In another study, in which rats were placed in voluntary running wheels at 10.5 mo or 18 mo of age, Goodrick and coworkers found no increase in longevity of the animals given access to running wheels (6). Four subsequent studies on specific-pathogen-free Long Evans rats given free access to voluntary running wheels have confirmed that wheel running increases average survival by  3 mo compared to sedentary freely eating controls (7)(8)(9)(10). However, in these studies the exercise had no effect on maximal life span. The differences between the results of these studies and that of Goodrick (5) are unexplained. However, as regular exercise was a normal, necessary component of everyday life throughout mammalian evolution, it is not reasonable to expect exercise to slow aging.
3 mo compared to sedentary freely eating controls (7)(8)(9)(10). However, in these studies the exercise had no effect on maximal life span. The differences between the results of these studies and that of Goodrick (5) are unexplained. However, as regular exercise was a normal, necessary component of everyday life throughout mammalian evolution, it is not reasonable to expect exercise to slow aging.
After a few months, rats generally lose interest in wheel running and markedly reduce or stop their running. In order to keep the animals running, the food intake of the voluntary runners in the studies on pathogen-free Long Evans rats was restricted to  92% of ad libitum intake (7). This slight food restriction prevents the abrupt decrease in running, perhaps by stimulating food-seeking behavior, but is too small to have an effect on longevity (7). With advancing age, voluntary running decreased gradually from
92% of ad libitum intake (7). This slight food restriction prevents the abrupt decrease in running, perhaps by stimulating food-seeking behavior, but is too small to have an effect on longevity (7). With advancing age, voluntary running decreased gradually from  6000 meters/24 h when the rats were young to 1000–1500 km/24 h at age 30 months (7)(8)(9)(10).
6000 meters/24 h when the rats were young to 1000–1500 km/24 h at age 30 months (7)(8)(9)(10).
Numerous studies have shown that DR significantly increases maximal longevity in rodents (11)(12)(13). Hypotheses that have been proposed to account for the life-prolonging effect of FR include growth retardation with maintenance of growth potential (14), prevention of excess fat accumulation (15), and a shift from cellular proliferation to maintenance and repair pathways (11)(16). Exercise has a number of effects that are similar to those of food restriction and that run counter to some of the changes that occur with aging in sedentary mammals (17)(18)(19)(20)(21)(22)(23)(24). This is particularly true in male rats, which are unusual in that, unlike most mammals, they do not increase their food intake to compensate for the increase in energy expenditure induced by exercise (7)(17)(18)(21)(22). As a consequence, male rats that exercise regularly are similar to FR rats in that they have growth retardation, a lower body fat content, and reduced availability of energy for cell proliferation compared to sedentary, freely eating animals (7)(17)(18)(21)(22). Male voluntary wheel-running rats are therefore a useful model for testing some of the hypotheses regarding the mechanisms by which FR extends maximal longevity.
In the studies in which voluntary wheel running did not increase maximal life span in male rats (despite growth retardation, reduced body fat, and decreased availability of energy for cell proliferation), the sedentary, paired weight control animals had a significant extension of maximal life span (7)(8). These animals had their food intake restricted (by  25% to 30%) to keep their body weights in the same range as those of the runners. Thus, the same relative energy deficit slowed aging when caused by FR, but not when caused by exercise. The food restriction also significantly decreased the incidence of malignancies, whereas exercise had no effect on the cause of death (7). One possible explanation for these divergent results is that FR extends maximal life span due to some consequence of decreased intake and/or metabolism of food other than growth retardation, reduced body fat, and decreased availability of energy for cell proliferation. Another is that a life-prolonging effect of decreased availability of energy for growth, fat synthesis, and cell proliferation is countered by some harmful effect of exercise, such as increased free radical production and oxidative tissue damage.
25% to 30%) to keep their body weights in the same range as those of the runners. Thus, the same relative energy deficit slowed aging when caused by FR, but not when caused by exercise. The food restriction also significantly decreased the incidence of malignancies, whereas exercise had no effect on the cause of death (7). One possible explanation for these divergent results is that FR extends maximal life span due to some consequence of decreased intake and/or metabolism of food other than growth retardation, reduced body fat, and decreased availability of energy for cell proliferation. Another is that a life-prolonging effect of decreased availability of energy for growth, fat synthesis, and cell proliferation is countered by some harmful effect of exercise, such as increased free radical production and oxidative tissue damage.
These possibilities were evaluated in studies in which male voluntary wheel running rats were either (a) food restricted to the same extent ( 30% below ad libitum) as the paired weight sedentary controls in the previous study, or (b) fed large amounts of antioxidants to protect against oxidative damage. The antioxidant-supplemented diet had no effect on longevity of the wheel runners, suggesting that increased oxidative stress caused by exercise was not adversely affecting longevity (25). In a study in which exercise and food restriction were combined, the survival curves of the FR runners and FR sedentary animals were virtually identical (10). This finding—that exercise does not interfere with the extension of maximal life span by food restriction—shows that exercise does not have a harmful effect that counters a life-prolonging effect of decreased availability of energy for cell proliferation. It provides evidence against the hypothesis that decreased availability of energy for a variety of biological processes, including growth, fat deposition, and cellular proliferation (11)(14)(15)(16), mediates the extension of maximal life span induced by food restriction. Thus, the information provided by the studies of the effects of exercise and FR in male rats favors the alternative hypothesis—that the life-extending effect of FR is not caused by the energy deficit per se, but results from some other consequence of decreased intake and metabolism of food.
30% below ad libitum) as the paired weight sedentary controls in the previous study, or (b) fed large amounts of antioxidants to protect against oxidative damage. The antioxidant-supplemented diet had no effect on longevity of the wheel runners, suggesting that increased oxidative stress caused by exercise was not adversely affecting longevity (25). In a study in which exercise and food restriction were combined, the survival curves of the FR runners and FR sedentary animals were virtually identical (10). This finding—that exercise does not interfere with the extension of maximal life span by food restriction—shows that exercise does not have a harmful effect that counters a life-prolonging effect of decreased availability of energy for cell proliferation. It provides evidence against the hypothesis that decreased availability of energy for a variety of biological processes, including growth, fat deposition, and cellular proliferation (11)(14)(15)(16), mediates the extension of maximal life span induced by food restriction. Thus, the information provided by the studies of the effects of exercise and FR in male rats favors the alternative hypothesis—that the life-extending effect of FR is not caused by the energy deficit per se, but results from some other consequence of decreased intake and metabolism of food.
Both paradigms, dietary restriction and exercise, have a number of technical considerations that have to be considered within an experimental context. The term "dietary restriction" is used to describe very different protocols of feeding, diet, duration, and onset. There is a consensus that malnutrition should be avoided, but little else. In comparing studies, it should be appreciated that the usual criterion for a dietary restriction study (i.e., a certain percentage reduction compared to ad libitum consumption) can result in large differences between studies because ad libitum (AL) consumption can vary so widely (26)(27)(28). Thus, the DR animals in one study may be larger than the AL animals in another study. In addition, studies started at 8 weeks of age have different physiological consequences from those started at 16 weeks of age (29). Diet composition (e.g., high fat or low fat) can also impact differently on particular endpoints, such as the incidences of certain tumors (30).
As DR is used to describe many paradigms, so is exercise. Treadmill running, voluntary wheel running, swimming, ladder climbing, and tail suspension, among others, have all been included as exercise. The duration of the exercise varies widely, as "chronic" exercise can last from 2 weeks to lifetime. The intensity of the paradigms also varies widely and is sometimes difficult to quantitate (e.g., for swimming, given some animals' tendency to float). Unfortunately, experimental paradigms to test some types of exercise, such as anabolic, are not easy to perform in rodent models. However, some techniques have been developed, such as weighed jackets (31), that make the studies possible, if difficult. Both exercise and dietary restriction are both paradigms that encompass wide variations in procedure. For comparisons across studies and extrapolation from rodents to primates, it would be useful to have a common metric which takes into account the important factors in the protocols.
| Studies in Nonhuman Primates |
|---|
|
|
|---|
Changes in body weight and body composition during adulthood are generally similar in humans and macaques. There are increases in lean tissue and body fat into early adulthood (32). Males are heavier than females, and they have more lean mass and lower percentage body fat than females. Body fat increases during middle age, and some individuals become noticeably obese. Excess body fat is predominantly located in the abdominal region for both sexes. During late adulthood, body weight and lean body mass decrease, and the decrease may be greater for males than for females (33).
Dietary restriction is the only intervention that increases average maximal life span in laboratory rodents. It is presently unknown whether DR increases maximal life span in nonhuman primates. Currently, studies from the four nonhuman primate groups (Universities of Wisconsin and Maryland, National Institute of Aging, and Wake Forest University) have not been of sufficient duration to derive firm conclusions regarding DR and longevity. We briefly examine the effects of caloric restriction and physical activity on body composition in nonhuman primates.
Colman and colleagues (34) examined the effect of long-term DR on body composition in female rhesus macaques. They studied the effects of 20–30% dietary restriction on adult rhesus monkeys (n = 30 females and n = 16 males) with assessments of body composition using dual energy x-ray absorptiometry at baseline and at 6, 12, and 18 months. After 18 months of DR, restricted animals reduced total and relative amounts of fat mass compared to control animals. Moreover, the DR males experienced relatively little or no change in appendicular skeletal muscle mass. This study suggests that the primary effect of DR is on reducing fat mass, whereas no deleterious effect on skeletal muscle mass was observed. It is presently unclear, however, whether these changes in body composition are related to increased longevity.
Cefalu and colleagues (35) performed a study of 30% DR in 32 adult monkeys with endpoints related to atherosclerosis, including glycated proteins, insulin, glucose, insulin sensitivity measures, and intraabdominal fat. Results showed a significant reduction of body weight and intraabdominal fat in restricted animals. The decline in intraabdominal fat corresponded to improvements in peripheral insulin sensitivity. Although issues of maximal life span were not addressed in this study, one may speculate that DR may partially exert some of its cardioprotective effects by influencing intermediate health outcomes such as insulin sensitivity and intraabdominal fat. Work from Cefalu and colleagues suggests that the effects of dietary restriction on these outcome variables (e.g., fatness, insulin sensitivity) would probably reduce the comorbidities associated with premature mortality and not influence the "rate of aging" per se.
Hansen and Bodkin (36) documented the effects of long-term (>10 years) dietary restriction (35–40%) on eight adult male rhesus monkeys that were maintained throughout life on a regimen to stabilize body weight. These eight DR monkeys were compared to 19 AL-fed, age-matched control monkeys. The monkeys were all aged >18 years. Dietary restriction prevented the development of obesity in older-aged monkeys (>60 yrs in human terms). In addition, all aspects of physical examination, including hair coat, muscle conformation, and oral hygiene were in excellent condition in these older-aged monkeys, attesting to their excellent nutritional status. This study demonstrated that dietary restriction maintained normal adult lean body mass and normal body fat (compared to the age-matched AL-fed monkeys) with body composition values for the DR monkeys within the range seen in younger animals. Thus, it appears that dietary restriction may prevent the age-related increase in obesity-associated comorbidities.
Dietary Restriction and Energy Expenditure in Nonhuman Primates
Lane and colleagues (37) performed studies of energy expenditure in DR monkeys (30% restricted) as compared to controls. There was no difference between restricted and control monkeys with respect to daily energy expenditure after adjustment for differences in fat-free mass. This suggests that DR reduces energy expenditure primarily by decreasing the quantity of the metabolically active tissue. With respect to changes in body composition, dietary restriction showed reductions in the absolute quantity of lean body mass, although the relative amounts (%) of lean and fat tissue remained similar. If DR were found to extend life span in monkeys, it would be of interest to examine whether a decrease in resting energy expenditure would be one mechanism mediating this effect.
DeLany and associates (38) examined the effects of dietary restriction on body composition and energy metabolism. This study compared six DR monkeys (approximately 35–40% DR from AL levels) to eight AL-fed control monkeys (age-matched). Caloric intake, energy expenditure (measured by the doubly labeled water technique), and body composition (estimated by the tritiated water dilution method) were determined. Results showed that total daily energy expenditure was reduced and that increases in fat mass were prevented in the restricted monkeys when compared to the AL-fed ones. Interestingly, a reduced energy expenditure persisted, even when data were statistically adjusted for the lower body weight. That is, energy expenditure was lower per unit of body mass. These results differ from those reported by Lane and colleagues (37), due potentially to differences in the magnitude of the caloric restriction paradigms.
Dietary Restriction and Physical Activity in Nonhuman Primates
The assessment of physical activity in free-living animals has represented a significant challenge to physiologists. The use of doubly labeled water to assess total daily energy expenditure may be a valuable tool to broaden our knowledge regarding the effects of physical activity and dietary restriction on life span. Adult rhesus monkeys appear to be most active in the morning and exhibit variable levels of physical activity during the light phase of the circadian cycle. Activity during the daytime tends to be associated with bouts of eating. Older adults spend less time in locomotion, and there is a marked decline in vertical movement by older monkeys (39). Obese monkeys are much less active than nonobese monkeys throughout the day (40).
Several studies have examined the effects of programmed exercise on body composition, but a direct comparison with DR is lacking. Cynomolgus monkeys fed an atherogenic diet and exercised on a treadmill weighed less than nonexercised controls on the same diet (41). The same species fed an atherogenic diet and exercised on a wheel weighed less and had less subcutaneous abdominal fat, but not visceral fat, than sedentary monkeys (42). Adult male baboons who were exercised on a wheel for  35 weeks had lower fasting plasma insulin levels despite no change in body weight (43). Generally, a large volume of exercise is required to alter body composition with animals not on DR. Less information is available regarding the effects of DR on measured physical activity in nonhuman primates. The effect of DR on physical activity is likely dependent on the severity of the restriction, the age at which the restriction is initiated, and the length of the DR period.
35 weeks had lower fasting plasma insulin levels despite no change in body weight (43). Generally, a large volume of exercise is required to alter body composition with animals not on DR. Less information is available regarding the effects of DR on measured physical activity in nonhuman primates. The effect of DR on physical activity is likely dependent on the severity of the restriction, the age at which the restriction is initiated, and the length of the DR period.
DeLany and colleagues (38) examined the effects of dietary restriction on physical activity. Measurements of physical activity in six of the DR monkeys using ultrasound were compared to eight lean young adult monkeys or age-matched AL-fed monkeys. Results showed that physical activity energy expenditure did not differ significantly between the DR group and the young weight-matched group.
Ramsey and colleagues (44) measured energy expenditure, activity, and body composition in 30 adult male rhesus monkeys using indirect calorimetry. Restricted animals were maintained at 70% of the caloric intake of controls. Results showed that at the 24- and 30-month assessments, nighttime energy expenditure was significantly reduced in the restricted animals after adjustment for lean body mass, whereas morning, afternoon, and total energy expenditures were not significantly different. No significant differences in physical activity were noted between treatment groups at any timepoint.
The experimental approach in rodents has been to implement dietary restriction in early life and use life span and terminal pathology as primary endpoints or to sacrifice groups at intermediate timepoints. In larger and longer-lived primate species, there is an opportunity to implement dietary restriction in mature animals and then, with regular assessments, track the appearance of risk factors and the development of disease culminating in death relative to fully fed controls. Clearly, the information is scanty at this point, with respect to the effects of DR on body composition and energy expenditure and its relationship to mortality in nonhuman primate species. However, given that these studies are underway and progressing, new information will be forthcoming.
In summary, the effects of DR on longevity in nonhuman primates is unknown. Dietary restriction, however, is effective in reducing total and central body fatness. The role of alterations in body composition as a modulator of life span are unclear at this point. Alterations in body composition by exercise and/or DR are probably effective in reducing obesity-related comorbidities associated with a physically inactive lifestyle. It is possible that alterations in body composition induced by caloric restriction and/or physical activity may play a role in reducing disease-related morbidities, but probably do not extend maximal longevity. It is also important to note that current studies have used different strategies for restricting calories (e.g., weight clamping or reducing intake based on individualized baseline caloric intakes). In future studies, a standardized approach should be adopted so that results among investigators can be appropriately compared. Similarly, different diets are being employed. Researchers should attempt to standardize the diets and the environment so that comparisons can be made across studies. The panel also noted the continuing value of the NIA-supported set-aside colonies of aged rhesus monkeys. We would suggest that this program should be enhanced, as well as encouraging the sharing of primate resources for aging research.
| Studies in Humans |
|---|
|
|
|---|
Some of the earliest works of exercise and mortality were conducted in the 1940s on London bus drivers, postal service workers, and civil servants (46)(47)(48)(49). The general notion advanced by these elegant studies was that both occupational and leisure time physical activity were associated with lower rates of cardiovascular disease. Paffenberger and colleagues conducted one of the first studies to attempt to quantify "years of life gained" from being physically active in the Harvard Alumni Study (50). Physical activity was assessed from 1962 and 1966 by asking participants about activities such as flights of stairs climbed, city blocks walked, and participation in different sports. This information provided an estimate of the energy expenditure in kilocalories per week expended. Individuals were reexamined in 1978. These investigators found a decline in mortality rates with increasing physical activity, with the benefit diminishing after an expenditure of approximately 3500 kcal per week. They estimated that the number of years of "added life" (truncated at 80 yrs) for individuals expending at least 2000 kcal per week would be approximately 2 years longer than for their more sedentary counterparts expending less than 500 kcal per week.
In a follow-up study (51), this group examined the relative contributions of vigorous and nonvigorous physical activity toward promoting longevity. In other words, does a similar caloric expenditure produced at different exercise intensities impact on longevity in a similar manner? Using Harvard Alumni data, these investigators found that vigorous, but not nonvigorous activity, significantly predicted mortality. This finding would argue in favor of vigorous activities that produce significant changes in maximal aerobic fitness (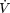 O2 max) as being the most favorable in prolonging life. A significant influence of participation in vigorous activity was also found in the U.S. Railroad Study (52). Men's physical activities were categorized based on their exercise intensity in 1957 and again in 1977. Mortality rates declined with increasing physical activity. When activity levels were categorized by vigorous, and light to moderate physical activity, only vigorous physical activity was significantly and inversely related to mortality rates. Again, this study reinforces the concept of vigorous physical activity as a modifier of longevity.
O2 max) as being the most favorable in prolonging life. A significant influence of participation in vigorous activity was also found in the U.S. Railroad Study (52). Men's physical activities were categorized based on their exercise intensity in 1957 and again in 1977. Mortality rates declined with increasing physical activity. When activity levels were categorized by vigorous, and light to moderate physical activity, only vigorous physical activity was significantly and inversely related to mortality rates. Again, this study reinforces the concept of vigorous physical activity as a modifier of longevity.
Pekkanen and colleagues (53) suggested that physical activity decreases mortality, but does not extend life span. Investigators classified 636 men into low and high physical activity levels. These investigators found that physically active men experienced greater survival after a 16-year follow-up period. At the end of the study, the oldest subjects approached 85 years of age. Interestingly, after this period of follow-up, the two survival curves tended to merge.
Several studies have examined the relationship between physical fitness and all-cause mortality. Physical fitness (i.e., time on treadmill or 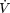 O2 max) is a biological attribute, whereas physical activity is a volitional behavior. Blair and colleagues (54) measured physical fitness using a maximal treadmill test to exhaustion in 10,244 men and 3,210 women aged 20 to 60+ years. They found a trend of declining mortality rates with increased physical fitness. In a follow-up study, they examined the effects of changes in physical fitness on mortality rates (55) after a mean interval between examinations of 4.9 years. The major conclusion from this report was that moving from being unfit to being fit was associated with a 60% reduction in mortality. There is a general consensus that individuals who are physically active or physically fit are less likely to experience premature mortality. The absence, however, of randomized controlled studies (probably due to cost and feasibility) precludes firm conclusions regarding the causal link between physical activity and mortality at this time. Nonetheless, the body of evidence suggests that physical activity and/or physical fitness probably exerts its effects in postponing mortality by reducing metabolic and cardiovascular disease risk.
O2 max) is a biological attribute, whereas physical activity is a volitional behavior. Blair and colleagues (54) measured physical fitness using a maximal treadmill test to exhaustion in 10,244 men and 3,210 women aged 20 to 60+ years. They found a trend of declining mortality rates with increased physical fitness. In a follow-up study, they examined the effects of changes in physical fitness on mortality rates (55) after a mean interval between examinations of 4.9 years. The major conclusion from this report was that moving from being unfit to being fit was associated with a 60% reduction in mortality. There is a general consensus that individuals who are physically active or physically fit are less likely to experience premature mortality. The absence, however, of randomized controlled studies (probably due to cost and feasibility) precludes firm conclusions regarding the causal link between physical activity and mortality at this time. Nonetheless, the body of evidence suggests that physical activity and/or physical fitness probably exerts its effects in postponing mortality by reducing metabolic and cardiovascular disease risk.
Exercise and Body Composition in Humans
A significant number of studies have examined the effect of physical activity on body composition in older individuals. The high level of interest in this topic is partly mediated by the recognition of: (a) age-related changes in physical activity, body composition, and their impact on health risk, and (b) the beneficial effect that increasing physical activity may have on improving cardiovascular and metabolic health in older individuals. This area of investigation, coupled with significant improvements in methods to measure energy expenditure (i.e., doubly labeled water) and body composition, have broadened our understanding of the relationship between physical activity and body composition.
Toth and colleagues (56) examined this area in an attempt to understand the effects of exercise on body composition and its potential for influencing longevity. The decline in physical activity with age and associated reduction in daily energy expenditure are thought to be important mediators of deleterious changes in body composition. Concomitant with the decline in physical activity, a loss of fat-free mass (specifically, skeletal muscle mass) and increase in fat mass are frequently observed. These age-related changes in body composition contribute to increased disease risk and reduced functional independence in elderly people. It is important to note, however, that these conclusions are primarily derived from cross-sectional observations. Thus, the "true" rate of age-related changes in body composition remains unclear.
Despite the absence of causal evidence that age-related changes in body composition are the result of a decline in physical activity, investigators have conducted exercise training experiments to examine its effects on body composition. In this section, we briefly consider exercise intervention studies that have focused on older men and women. We considered the effects of both aerobic and resistance exercise training on body composition. The rationale for separating these two exercise paradigms is that their effects on body composition may differ: aerobic exercise primarily reduces body fat by promoting a negative energy imbalance, whereas resistance exercise primarily increases fat-free mass by stimulating skeletal muscle growth. In a recent review of exercise studies in older people (56), several conclusions were drawn: (a) aerobic exercise is effective in reducing fat mass, but has little effect on fat-free mass; (b) the magnitude of aerobic exercise-induced changes in fat mass is related to the total number of sessions in the exercise program; and (c) resistance exercise is effective in reducing fat mass and increasing fat-free mass. It should be noted, however, that none of these studies directly compared these changes with those induced by caloric restriction.
Toth and associates (56) noted that aerobic exercise was effective in reducing body fat in studies that did not instruct patients to maintain their body weight. The effect of aerobic exercise to reduce body fat is not unexpected, although the mechanism by which this change occurs is unclear. Aerobic-induced changes in body fat were not related to the method used to assess body composition. However, the change in fat mass was primarily determined by the total number of sessions in the aerobic exercise program. That is, as the number of exercise sessions increased, the amount of fat loss increased. Thus, the length of exposure to the exercise stimulus or the absolute caloric expenditure of the exercise program may be an important determinant of the amount of fat lost during aerobic exercise in older individuals.
It is generally thought that aerobic exercise reduces body fat stores by promoting a negative energy imbalance (i.e., energy expenditure exceeds energy intake). Specifically, aerobic exercise increases daily energy expenditure in older individuals through the direct energetic cost of the exercise and possibly by increasing resting energy expenditure. Recent findings (57), however, showed that 8 weeks of high-intensity aerobic exercise did not increase daily energy expenditure in older men and women because of compensatory decreases in physical activity energy expenditure during nonexercising time. Thus, the effect of aerobic exercise on body fat stores may be mediated by changes in energy intake, rather than changes in energy expenditure. Further studies are needed to delineate the mechanism by which aerobic exercise promotes a reduction in adiposity. Understanding the mechanism of exercise-induced changes in body composition will allow the refinement of exercise prescriptions (i.e., intensity and duration) to optimize reductions in body fat.
Aerobic exercise does not appear to alter fat-free mass in older individuals. Only 7 out of 35 studies (57) considered showed increased fat-free mass, and this increase did not exceed 1 kg in most studies. Changes in the hydration of fat-free mass due to increased glycogen storage may account for these minimal changes in fat-free mass, although this has not been directly tested. The lack of an effect of aerobic exercise on fat-free mass is not surprising, however, considering that this mode of exercise does not provide a significant anabolic stimulus to promote muscle growth. Recently, a randomized clinical trial of 6-month duration confirmed that resistance training increases fat-free mass, whereas aerobic training has no discernible effect (58). These results should not, however, be taken as evidence that aerobic exercise has no influence on fat-free mass. The possibility should be considered that aerobic training may be effective in attenuating the reduction in fat-free mass that results from inactivity and other hormonal and lifestyle factors. That is, aerobic exercise may provide an adequate stimulus to maintain fat-free mass with age. This hypothesis, however, has yet to be systematically tested.
Resistance training reduced fat mass in 15 out of 25 studies (56). On average, in studies which found a reduction in fat mass with resistance exercise, the loss was similar to those changes induced by aerobic exercise (resistance exercise: –1.7 ± 0.4 kg vs aerobic exercise: –1.9 ± 0.8 kg). This is somewhat surprising considering that the direct energetic cost of resistance exercise is smaller compared to that of aerobic exercise. The possibility that resistance training may increase daily energy expenditure by increasing the quantity of fat-free mass should be considered (59). These studies have mainly been confined to the examining of changes in resting metabolic rate. This is understandable, given that resting metabolic rate is a large component of daily energy expenditure. Theoretically, even small changes in resting metabolic rate may significantly affect the regulation of body weight and body composition over extended periods of time (59).
The effects of resistance training on resting metabolic rate are controversial. Broeder and colleagues (60) found no change in resting metabolic rate in 13 young male volunteers following 12 weeks of high-intensity training despite a 2 kg increase in fat-free mass. Given that the published regression equations in the literature, which show an increase in resting metabolic rate of approximately 25–45 kcal per day for each kilogram of fat-free mass (61), it may seem surprising that no increase in resting metabolic rate was found. Van Etten and coworkers (62) examined changes in energy expenditure in 21 younger males after a 12-week weight training program. Sleeping metabolic rate was measured in a respiration chamber. Weight training increased fat-free mass (1.1 ± 1.3 kg) and decreased fat mass (2.3 ± 1.5 kg). The investigators did not find any change in sleeping metabolic rate.
Pratley and associates (63) examined changes in resting metabolic rate following 16 weeks of resistance training in 13 older men. The use of resistance training in elderly people is particularly interesting because of the age-related decline in energy expenditure. Resting metabolic rate increased by approximately 8%, or 120 kcal per day. Although fat-free mass increased during the training program (1.6 kg), the increase in resting metabolic rate persisted after control for changes in fat-free mass. This finding suggests that the elevation in resting metabolic rate was due to both the increased quantity and metabolic activity of fat-free mass. The investigators speculated that the increase in resting metabolic rate was related to the increase in plasma levels of norepinephrine. However, they could not rule out the possibility that the elevated resting metabolic rate was due to the residual effect of the last bout of exercise, given that the resting metabolic rate measurement was performed 22 to 24 hr following the last training session. Both Campbell (64) and Trueth (65) and colleagues also found increases in resting metabolic rate in older men and women after chronic resistance training. These increases approached 7 to 10%, or approximately 100 to 150 kcal per day. Interestingly, in each of these studies, resting metabolic rate remained elevated after controlling for the increase in fat-free mass. These findings suggest that resistance training stimulates resting metabolic rate, particularly in older individuals, through a mechanism that is independent of changes in fat-free mass. Thus, resistance training may be a viable intervention to elevate daily energy expenditure and potentially reduce fat mass via its effects on body composition.
Caloric Restriction and Body Composition in Humans
Body weight remains stable when adult subjects are in zero energy balance. Negative energy balance, or DR, leads to losses of tissue energy pools and a decline in body mass. Negative energy balance leads to an exponential loss in body weight, with a rapid "early" phase lasting 5–10 days and a slower "late" phase thereafter. Protein balance, a component of energy balance, shows a similar pattern (66). Energy–nitrogen balance studies indicate that a relatively large proportion of early weight loss is extracellular fluid and protein. "Later" weight loss consists primarily of fat or triglycerides. Long-term (e.g., 2–4 months) weight loss in general consists of about 70–75% fat, and the remainder is fat-free mass (66)(67)(68)(69).
Although data on this issue are limited, relative fat-free mass loss with dieting may be greater in men than in women and in lean subjects than in subjects who are obese (70). Most weight-loss studies have concentrated on body composition changes in obese women (71). Some studies, however, have examined, in a controlled fashion, body composition changes in obese men and in lean men and women. Several independent factors also influence the rate and composition of weight loss in addition to duration of negative nutrient balance, gender, and initial body mass. These include:
• Dietary protein: Diets inadequate in protein, particularly high-quality protein, are associated with significant negative nitrogen balance and excessive lean mass loss (72)(73)(74).
• Total energy intake: Very low caloric intakes are associated with rapid weight loss, particularly with losses of total body protein. Severe negative nitrogen balance, body cell mass depletion, and rapid weight loss accompany total fasting.
• Diet composition: Diets low in carbohydrate (and thus high in fat and/or protein) tend to produce early diuresis associated with ketosis. However, net rates of long-term fat loss are similar in high- and low-carbohydrate diets.
• Physical activity level: Either alone or in combination with dieting, increased levels of physical activity may moderate the proportions of fat and lean tissue loss produced by negative energy balance (69)(70).
Several key issues remain in this area. First, information is sparse on changes in compartments other than "fat" and "fat-free mass." For example, there is some, but limited information on changes in skeletal muscle and visceral adipose tissue mass with weight loss and/or physical activity. Second, most research, up to now, has examined the effects of weight loss in obese women; information is limited for obese men and lean men and women. There is also limited information on how elderly subjects respond to negative energy balance in terms of body composition change. Third, the longest body composition studies with caloric restriction extend out to several months. Very little information is available on long-term effects of caloric restriction on body compartments.
Consistent with the goal of our panel, we offer several general recommendations for future research in nonprimate, nonhuman primate, and human studies:
| Recommendations |
|---|
|
|
|---|
Recommendations in Nonhuman Primate Studies
Recommendations in Human Studies
 20–25 years; (b) subjects discontinuing smoking; (c) postpartum women; (d) menopausal women. These individuals often show phenotypic predictors of long-term weight gain, including low resting metabolic rate, high respiratory quotient, and low physical activity level.
20–25 years; (b) subjects discontinuing smoking; (c) postpartum women; (d) menopausal women. These individuals often show phenotypic predictors of long-term weight gain, including low resting metabolic rate, high respiratory quotient, and low physical activity level. | Acknowledgments |
|---|
| References |
|---|
|
|
|---|
This article has been cited by other articles: (Search Google Scholar for Other Citing Articles)
 |
D. Nemet, P. H. Connolly, A. M. Pontello-Pescatello, C. Rose-Gottron, J. K. Larson, P. Galassetti, and D. M. Cooper Negative energy balance plays a major role in the IGF-I response to exercise training J Appl Physiol, January 1, 2004; 96(1): 276 - 282. [Abstract] [Full Text] [PDF] |
||||
 |
J. M. LaMothe, R. T. Hepple, and R. F. Zernicke Selected Contribution: Bone adaptation with aging and long-term caloric restriction in Fischer 344 x Brown-Norway F1-hybrid rats J Appl Physiol, October 1, 2003; 95(4): 1739 - 1745. [Abstract] [Full Text] [PDF] |
||||
 |
M.-M. G. Wilson and J. E. Morley Invited Review: Aging and energy balance J Appl Physiol, October 1, 2003; 95(4): 1728 - 1736. [Abstract] [Full Text] [PDF] |
||||
 |
H. T. Blumenthal The Aging-Disease Dichotomy: True or False? J. Gerontol. A Biol. Sci. Med. Sci., February 1, 2003; 58(2): M138 - 145. [Full Text] [PDF] |
||||
 |
J. E. Morley Anorexia and Weight Loss in Older Persons J. Gerontol. A Biol. Sci. Med. Sci., February 1, 2003; 58(2): M131 - 137. [Full Text] [PDF] |
||||
 |
J. E. Morley Editorial: Hot Topics in Geriatrics J. Gerontol. A Biol. Sci. Med. Sci., January 1, 2003; 58(1): M30 - 36. [Full Text] [PDF] |
||||
 |
J. E. Morley Editorial: Citations, Impact Factor, and the Journal J. Gerontol. A Biol. Sci. Med. Sci., December 1, 2002; 57(12): M765 - 769. [Full Text] [PDF] |
||||
 |
A. Turturro, P. Duffy, B. Hass, R. Kodell, and R. Hart Survival Characteristics and Age-Adjusted Disease Incidences in C57BL/6 Mice Fed a Commonly Used Cereal-Based Diet Modulated by Dietary Restriction J. Gerontol. A Biol. Sci. Med. Sci., November 1, 2002; 57(11): B379 - 389. [Abstract] [Full Text] [PDF] |
||||
 |
A. Fisher and J. E. Morley Editorial: Antiaging Medicine: The Good, the Bad, and the Ugly J. Gerontol. A Biol. Sci. Med. Sci., October 1, 2002; 57(10): M636 - 639. [Full Text] [PDF] |
||||
 |
D. Hamerman Molecular-Based Therapeutic Approaches in Treatment of Anorexia of Aging and Cancer Cachexia J. Gerontol. A Biol. Sci. Med. Sci., August 1, 2002; 57(8): M511 - 518. [Abstract] [Full Text] [PDF] |
||||
 |
R. L. Walford, D. Mock, R. Verdery, and T. MacCallum Calorie Restriction in Biosphere 2: Alterations in Physiologic, Hematologic, Hormonal, and Biochemical Parameters in Humans Restricted for a 2-Year Period J. Gerontol. A Biol. Sci. Med. Sci., June 1, 2002; 57(6): B211 - 224. [Abstract] [Full Text] |
||||
 |
J. E. Morley Editorial: Drugs, Aging, and the Future J. Gerontol. A Biol. Sci. Med. Sci., January 1, 2002; 57(1): M2 - 6. [Full Text] [PDF] |
||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| HOME | HELP | FEEDBACK | SUBSCRIPTIONS | ARCHIVE | SEARCH | TABLE OF CONTENTS |
|---|
| All GSA journals | The Gerontologist |
| Journals of Gerontology Series B: Psychological Sciences and Social Sciences | |